

The fourth electron is in the p orbital that will form the pi bond. In ethene, each hydrogen atom has one unpaired electron and each carbon is sp 2 hybridized with one electron each sp 2 orbital. The diagram below shows the bond lengths and hydrogen-carbon-carbon bond angles of ethene :Īccording to valence bond theory, two atoms form a covalent bond through the overlap of individual half-filled valence atomic orbitals, each containing one unpaired electron. In order for the unhybridized p orbitals to successfully overlap, the CH 2 must be coplanar: therefore, C 2H 4 is a planar molecule and each bond angle is about 120 degrees. Each carbon atom is of the general arrangement AX 3, where A is the central atom surrounded by three other atoms (denoted by X) compounds of this form adopt trigonal planar geometry, forming 120 degree bond angles. Valence Shell Electron Pair Repulsion (VSEPR) Theory is used to predict the bond angles and spatial positions of the carbon and hydrogen atoms of ethene and to determine the bond order of the carbon atoms (the number of bonds formed between them). The remaining unhybridized p orbitals on the carbon form a pi bond, which gives ethene its reactivity. Ethene consists of two sp 2-hybridized carbon atoms, which are sigma bonded to each other and to two hydrogen atoms each. In nature, it is released in trace amounts by plants to signal their fruits to ripen. With nitrogen, however, there are five rather than four valence electrons to account for, meaning that three of the four hybrid orbitals are half-filled and available for bonding, while the fourth is fully occupied by a (non-bonding) pair of electrons.Ĭ 2H 4, also known as ethylene or ethene, is a gaseous material created synthetically through steam cracking. Just like the carbon atom in methane, the central nitrogen in ammonia is sp 3-hybridized. The sp 3 bonding picture is also used to described the bonding in amines, including ammonia, the simplest amine. In chapter 3 we will learn more about the implications of rotational freedom in sigma bonds, when we discuss the ‘conformation’ of organic molecules. This means, in the case of ethane molecule, that the two methyl (CH 3) groups can be pictured as two wheels on a hub, each one able to rotate freely with respect to the other.
C2H2 VSEPR FREE
All of these are sigma bonds.īecause they are formed from the end-on-end overlap of two orbitals, sigma bonds are free to rotate.
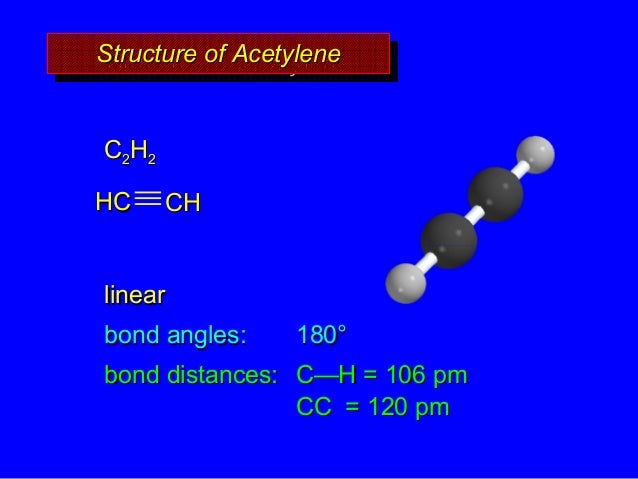
The carbon-carbon bond, with a bond length of 1.54 Å, is formed by overlap of one sp 3 orbital from each of the carbons, while the six carbon-hydrogen bonds are formed from overlaps between the remaining sp 3 orbitals on the two carbons and the 1 s orbitals of hydrogen atoms.

Both carbons are sp 3-hybridized, meaning that both have four bonds arranged with tetrahedral geometry. In the ethane molecule, the bonding picture according to valence orbital theory is very similar to that of methane.


 0 kommentar(er)
0 kommentar(er)
